Tag: biosimilars

- Dec, 4 2025
- Comments 15
Biologic Patent Protection: When Biosimilars Can Enter the U.S. Market
Biologic drugs in the U.S. enjoy 12 years of market exclusivity before biosimilars can enter, thanks to FDA rules and patent thickets. This delays competition, keeps prices high, and leaves patients paying more than those in other countries.
Read More
- Dec, 1 2025
- Comments 10
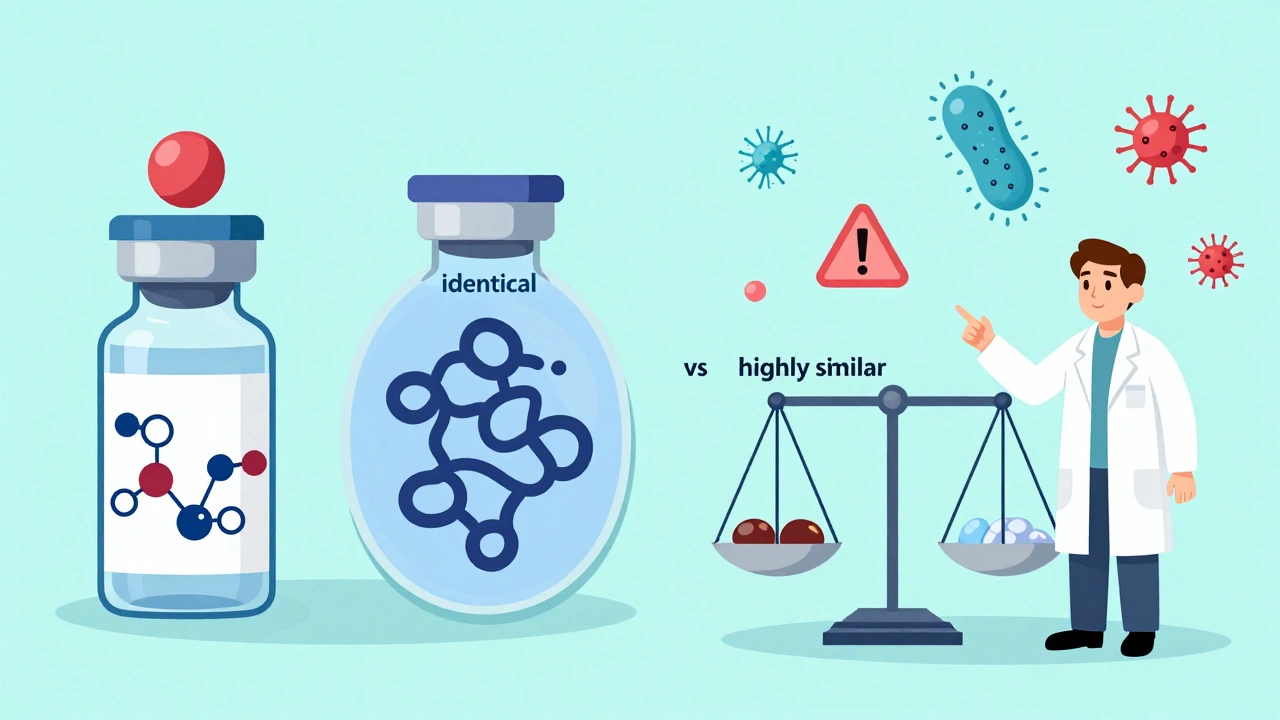
Provider Education: Understanding Biosimilar Differences
Biosimilars are not generics. They're complex biological drugs that require special understanding. Learn how they differ, why provider education matters, and how to confidently use them in practice.
Read More

- Nov, 12 2025
- Comments 11
Insurance Changes and Generic Switching: Navigating Formulary Updates in 2025
In 2025, Medicare Part D formulary changes are pushing patients toward generics and biosimilars to lower costs. Learn how these shifts affect your prescriptions, what your rights are, and how to avoid unexpected price hikes.
Read More