Provider Education: Understanding Biosimilar Differences
 Dec, 1 2025
Dec, 1 2025
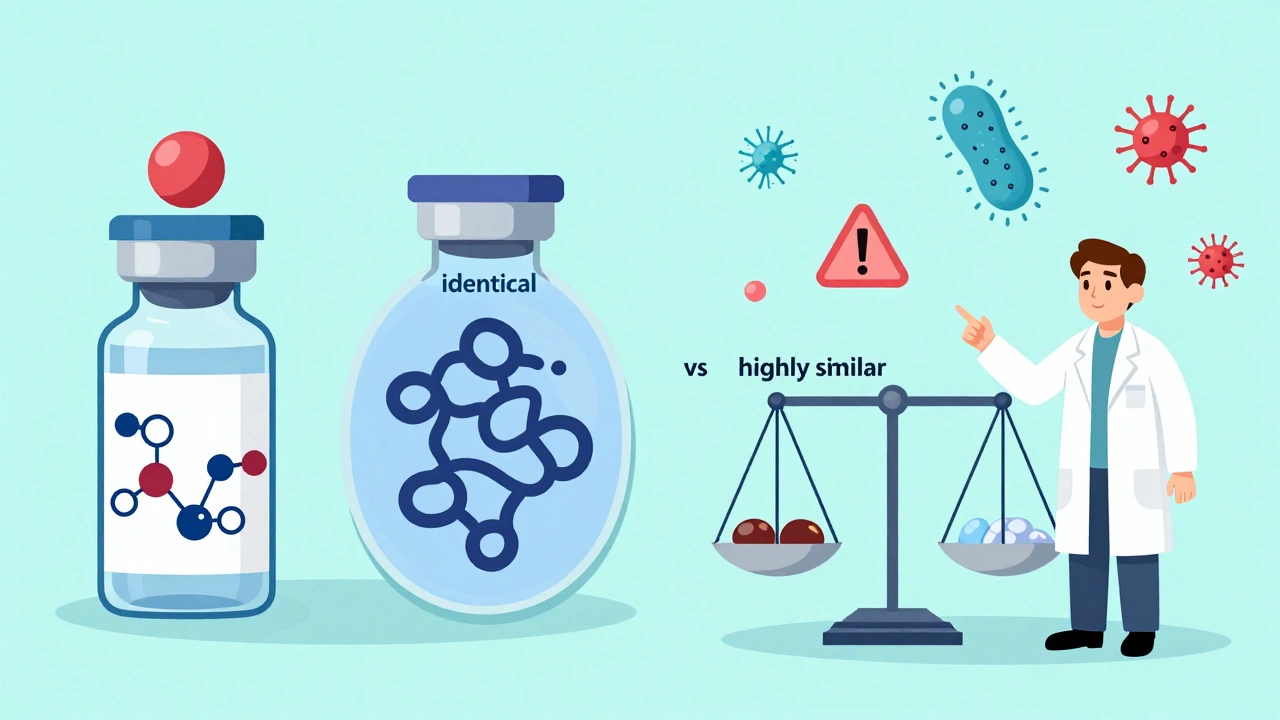
Most doctors and pharmacists know what a generic drug is. But when it comes to biosimilars, confusion is still widespread-even among experienced providers. A 2021 survey found that only 38% of U.S. physicians felt extremely familiar with the FDA’s definition of a biosimilar. That’s not because they’re untrained. It’s because biosimilars aren’t generics. They’re something entirely different-and understanding that difference is critical for patient safety and treatment success.
What Exactly Is a Biosimilar?
A biosimilar is a biological product that is highly similar to an already-approved reference biologic. It’s not an exact copy, but it must have no clinically meaningful differences in safety, purity, or potency. The FDA requires manufacturers to prove this through thousands of lab tests, animal studies, and at least one clinical trial in humans. Unlike generics, which are chemically identical to their brand-name counterparts, biosimilars are made from living cells-like yeast, bacteria, or mammalian cells. That means tiny variations in manufacturing can affect structure and behavior, even if the final product works the same way in the body.Think of it like this: two handmade leather jackets might look identical, but one uses different stitching techniques or tanning methods. Both keep you warm. But if one causes an allergic reaction in someone with sensitive skin, you need to know why. That’s why biosimilars require more testing than generics. The FDA mandates up to 100 times more analytical data for biosimilars than for traditional generics.
Biosimilars vs Generics: The Key Differences
| Feature | Generic Drugs | Biosimilars |
|---|---|---|
| Chemical Structure | Small molecule, identical to brand | Large, complex protein, highly similar but not identical |
| Manufacturing Process | Chemical synthesis, easily replicated | Live cell culture, sensitive to changes |
| Testing Required | Bioequivalence study only | Analytical, non-clinical, and clinical studies |
| Interchangeability | Automatic substitution allowed | Requires extra FDA approval for substitution |
| Indication Extrapolation | Not applicable | Permitted with strong scientific justification |
One major point of confusion is interchangeability. Not all biosimilars are interchangeable. Only those that have passed additional studies-proving that switching back and forth between the reference product and biosimilar doesn’t increase risk-can be substituted at the pharmacy without a prescriber’s permission. As of late 2023, only six biosimilars in the U.S. had received this designation. The rest require a specific prescription.
Why Provider Education Matters
A 2017 study of oncology staff showed that after just 12 training sessions over four months, provider confidence in biosimilar efficacy jumped from 40% to 92%. That’s not a small gain. That’s life-changing for patients who might otherwise delay treatment due to cost.But education isn’t just about efficacy. It’s about trust. Many patients hear “biosimilar” and think “cheap version.” If providers don’t understand the science themselves, they can’t explain it clearly. A 2021 survey found that only 22% of U.S. pharmacists felt very confident explaining biosimilar differences to patients. That’s a problem. When patients are confused, they refuse treatment. At UCSF Medical Center, pharmacist-led education cut prescribing hesitancy among oncologists from 58% to 12% in six months.
Even more troubling: 57% of providers worry about using biosimilars for conditions not directly studied in trials. This is called extrapolation. For example, if a biosimilar is approved for rheumatoid arthritis based on one study, the FDA may allow it to be used for psoriatic arthritis too-if the mechanism of action is the same and the disease pathways are similar. It’s scientifically valid, but many providers still hesitate.
Where Adoption Stands Today
Biosimilars are saving money-real money. Medicare Part B data from 2022 shows biosimilars cost 28% less on average than their reference biologics. The Congressional Budget Office estimates they could save the U.S. healthcare system $150 billion over the next decade.But adoption isn’t equal across specialties. Rheumatologists lead the way, with 68% using biosimilars regularly. Oncologists are close behind at 52%. But endocrinologists? Only 29%. And insulin biosimilars have been available since 2015. Why the lag?
Part of it is EHR issues. A 2022 survey found that 78% of U.S. hospitals struggle to document biosimilar use correctly in electronic health records. Some systems don’t even have a dropdown for biosimilars. Providers end up typing it manually, which leads to billing errors and tracking problems. At one major hospital, nurses reported spending up to 15 extra minutes per patient just to get the right code into Epic.
What Providers Need to Know
There are four core areas every provider should understand:- Immunogenicity - Biosimilars can trigger immune responses, just like the reference product. But differences in manufacturing mean the risk profile isn’t always identical. Monitoring for antibodies is still necessary.
- Extrapolation - Just because a biosimilar works in one condition doesn’t mean it automatically works in all conditions the reference product treats. But the FDA approves extrapolation when the science supports it. Ask: Is the mechanism of action the same? Are the disease pathways similar?
- Interchangeability - Know which biosimilars in your practice have this designation. Only those can be substituted without a new prescription. Check the FDA’s Purple Book regularly.
- Documentation - Your EHR must accurately record which product was given. If you prescribe a biosimilar, don’t just write “adalimumab.” Write “adalimumab-atto” or “adalimumab-adaz.”
There’s also the issue of patient communication. A 2022 survey from the ArthritisPower registry found that 34% of rheumatology patients felt confused when switched to a biosimilar. Why? Because their provider didn’t explain it well. Patients need to hear: “This is not a cheaper version. It’s a scientifically proven alternative with the same results and safety profile.”

Where to Get Reliable Education
The FDA has created a free, comprehensive Teaching Resource Guide with 12 modules covering everything from manufacturing to billing. It’s available in nine languages and meets accessibility standards. Medical schools, pharmacy programs, and nursing schools across the country are starting to use it.But education shouldn’t stop at the classroom. Hospitals that succeed have dedicated clinical pharmacists leading ongoing training. At the University of Pittsburgh Medical Center, they used a three-phase approach: four weeks of foundational learning, six weeks of specialty-specific application, and ongoing support. Within six months, 89% of providers reported high confidence in using biosimilars.
Don’t rely on manufacturer materials. The FDA’s resources are independent, evidence-based, and updated regularly. The Arthritis Foundation, the American College of Rheumatology, and the Alliance for Safe Biologic Medicines also offer free provider toolkits with case studies, FAQs, and patient handouts.
The Future Is Here-Are You Ready?
By 2027, biosimilars are projected to make up 45% of the biologics market. That’s not a distant forecast. It’s happening now. Insulin, Humira, Enbrel, Herceptin-all have biosimilar alternatives. And more are coming.The biggest barrier isn’t cost. It’s knowledge. Providers who understand biosimilars aren’t just keeping up-they’re leading the charge toward more affordable, accessible care. Those who don’t? They risk misinformed patients, delayed treatments, and avoidable errors.
You don’t need to be a scientist to use biosimilars. But you do need to know the basics. Start with the FDA’s guide. Talk to your pharmacy team. Ask your hospital if they have a biosimilar education program. If they don’t, start one.
Patients are counting on you to get it right.
Are biosimilars the same as generics?
No. Generics are chemically identical copies of small-molecule drugs. Biosimilars are highly similar versions of complex biological drugs made from living cells. They require far more testing because their structure can’t be exactly replicated. While generics can be substituted automatically, biosimilars need special approval for interchangeability.
Can biosimilars be substituted without a new prescription?
Only if they’re designated as “interchangeable” by the FDA. As of late 2023, only six biosimilars in the U.S. have this status. Even then, state laws vary. Some states require prescriber notification, others don’t. Always check your state’s substitution rules before switching.
Why do some providers hesitate to use biosimilars?
Common concerns include fear of immunogenicity, uncertainty about extrapolation (using a biosimilar for an untested condition), and lack of familiarity with EHR documentation. Many also worry about patient confusion or backlash. But studies show that with proper education, provider confidence rises dramatically-and patient outcomes stay the same.
Do biosimilars work as well as the original biologic?
Yes. The FDA requires biosimilars to show no clinically meaningful differences in safety, purity, or potency. Over 100 clinical studies have confirmed this across conditions like rheumatoid arthritis, cancer, and inflammatory bowel disease. Real-world data from Europe, where biosimilars have been used for over a decade, supports the same conclusion.
How can I learn more about biosimilars?
Start with the FDA’s free Teaching Resource Guide, which includes 12 modules on biosimilar science, regulation, and clinical use. Clinical pharmacists in your hospital can also be valuable resources. Professional societies like the American College of Rheumatology and the American Society of Clinical Oncology offer provider toolkits and webinars. Many of these are free and updated annually.
Fern Marder
December 1, 2025 AT 18:37Okay but can we talk about how wild it is that we’re still having this conversation in 2024? 🤯 I work in a hospital and half the nurses still call biosimilars ‘the cheap stuff.’ Like… no. It’s not cheap. It’s *clever*. And if your EHR doesn’t even have a dropdown for it, that’s a system failure, not a provider failure. Fix the tech first, then the training. 🙃
Carolyn Woodard
December 3, 2025 AT 05:38The ontological distinction between small-molecule generics and complex protein biosimilars reveals a fundamental epistemological gap in contemporary pharmacovigilance frameworks. The reductionist paradigm of chemical equivalence fails to account for post-translational heterogeneity inherent in biologics, rendering the term ‘identical’ semantically incoherent in this context. Consequently, regulatory paradigms must evolve beyond bioequivalence metrics toward structural fidelity indices derived from multi-omic analytics.
Allan maniero
December 3, 2025 AT 16:51I’ve seen this play out in the UK too - rheumatology units are rocking biosimilars, but endocrinology? Still clinging to the original like it’s a family heirloom. Honestly, it’s less about science and more about habit. I had a colleague who refused to switch a patient to an insulin biosimilar because ‘I’ve always prescribed Humalog.’ I said, ‘So you’ve been prescribing a brand name because you like the logo?’ He paused. Then he switched three patients the next week. Sometimes you just need to ask the right question.
Anthony Breakspear
December 5, 2025 AT 10:56Let’s be real - the real villain here isn’t the provider, it’s the EHR vendors. Epic, Cerner, all of ‘em. They’re sitting on billions, but they can’t add a simple dropdown for ‘adalimumab-adaz’? That’s not incompetence, that’s greed. Why? Because if they make it easy, hospitals won’t need to pay for ‘custom integration services’ at $200/hour. Meanwhile, nurses are typing ‘HUMIRA biosimilar’ into free-text fields and billing gets screwed. Fix the software, not the staff. 🤷♂️
Zoe Bray
December 6, 2025 AT 02:48It is imperative that healthcare institutions institute standardized, evidence-based educational protocols regarding biosimilar therapeutics, as outlined by the Food and Drug Administration’s Teaching Resource Guide. The absence of institutionalized training constitutes a systemic deficiency in clinical governance, potentially exposing patients to unnecessary risk through provider uncertainty. Formal competency assessments should be mandated for all prescribers and pharmacists involved in biologic therapy management.
Girish Padia
December 6, 2025 AT 18:41People act like biosimilars are some big conspiracy. Nah. Big pharma just wants you to pay $2k for a shot when it costs $50 to make. If you’re not using them, you’re basically stealing from Medicare and your own patients. Stop being lazy and read the FDA docs. Your patients don’t care about your ego.
Saket Modi
December 6, 2025 AT 22:59Ugh. Another one of these ‘education is the answer’ rants. We’ve been hearing this since 2015. Where’s the money? Where’s the time? I got 3 mins per patient and a 100-page chart. Tell me how I’m supposed to explain protein folding between vitals and the next appointment. 😴
Chris Wallace
December 8, 2025 AT 05:35I think the most overlooked part is how patient trust gets damaged not by the drug, but by the silence around it. I had a patient cry last month because she thought switching to a biosimilar meant she was getting ‘second-best care.’ She didn’t know the science - and her provider never explained it. No one yelled. No one panicked. Just… silence. And now she’s terrified of every injection. It’s not about jargon. It’s about listening. And then saying, ‘This isn’t a downgrade. It’s an upgrade in access.’
Sheryl Lynn
December 9, 2025 AT 23:48Let’s be honest - if you’re still conflating biosimilars with generics, you’re not just behind the curve, you’re on a different continent. The fact that we’re still debating this in 2024 is a tragic testament to the mediocrity of medical education. Real innovation isn’t just about molecules - it’s about intellectual courage. And frankly, most providers are still wearing 2008-era thinking with 2024’s price tag. 🤦♀️
John Biesecker
December 11, 2025 AT 08:31so like… i just read the fda guide and holy smokes it’s actually kinda chill? like, not boring at all. and the part about how they test biosimilars like 100x more than generics? mind blown. also i typed ‘adalimumab-atto’ wrong in my chart yesterday and got flagged by billing. oops. 😅 but now i know. thanks for the post, this is actually super helpful.