The 80-125% Rule: Understanding Bioequivalence Confidence Intervals for Generic Drugs
 Dec, 5 2025
Dec, 5 2025
When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how do regulators know it’s truly the same? The answer lies in something called the 80-125% rule-a quiet but powerful standard that ensures thousands of generic drugs are safe and effective without requiring new clinical trials every time.
What the 80-125% Rule Actually Means
Many people think the 80-125% rule means generic drugs can contain anywhere from 80% to 125% of the active ingredient compared to the brand-name drug. That’s not true. The rule doesn’t refer to the amount of drug in the tablet. It refers to how much of that drug actually gets into your bloodstream-and how fast.
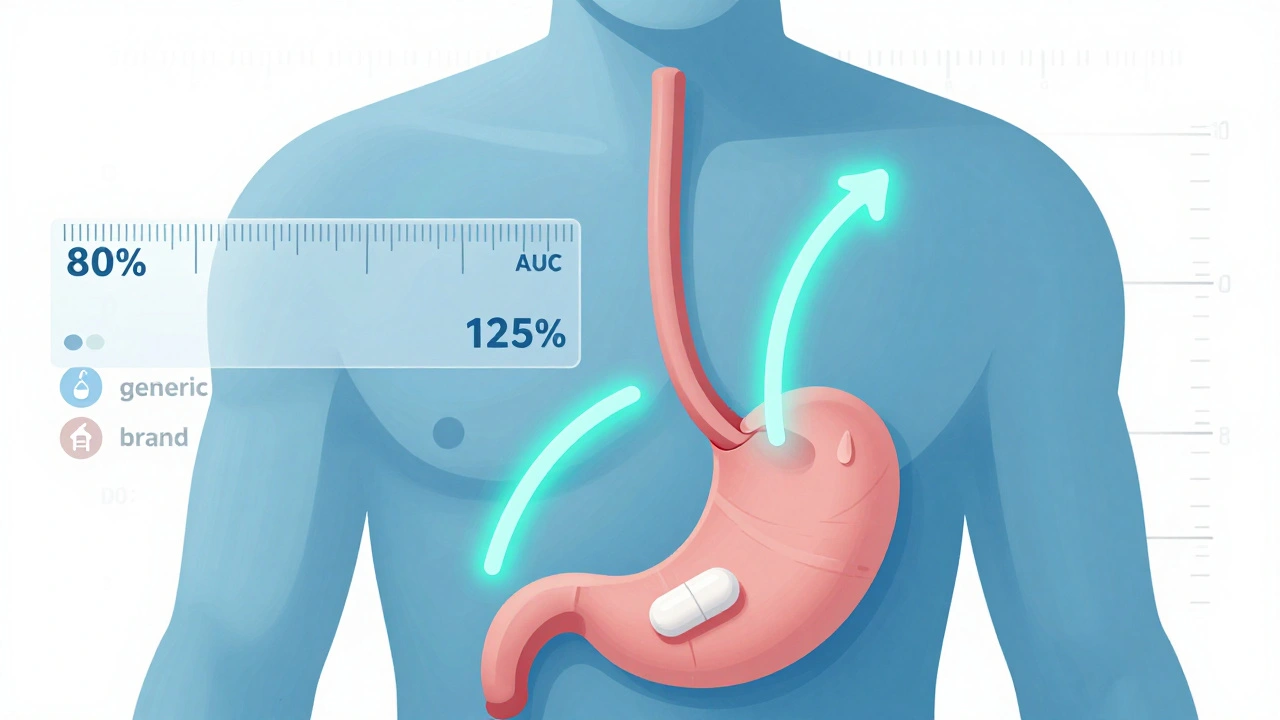
Regulators look at two key measurements: AUC (Area Under the Curve), which tells you how much of the drug your body absorbs over time, and Cmax (maximum concentration), which shows how quickly it reaches its highest level in your blood. These numbers come from clinical studies with healthy volunteers, usually in a crossover design where each person takes both the brand and generic versions at different times.
After measuring these values, scientists transform the data using a logarithmic scale. On this scale, the acceptable range becomes symmetric: from -0.2231 to +0.2231. When you convert those numbers back to percentages, you get 80% and 125%. The 90% confidence interval of the ratio between the generic and brand drug’s geometric means must fall entirely within that range. If it does, the two products are considered bioequivalent.
This isn’t about guesswork. It’s a statistically sound method developed after years of research and debate. In 1986, the FDA held a public hearing where experts agreed that differences smaller than 20% in absorption are unlikely to cause real-world problems for most patients. That’s why the 80-125% range became the global standard.
Why a 90% Confidence Interval? Not 95%
You might wonder why regulators use a 90% confidence interval instead of the more common 95%. It’s a deliberate choice. Using a 90% CI allows for a 5% error margin on each side-totaling 10%-which balances the need for strict standards with practical study design.
If they used a 95% CI, the range would be wider, making it harder to prove equivalence. That could block good generics from reaching the market. A 90% CI is narrow enough to catch meaningful differences but wide enough to avoid rejecting products that are clinically equivalent. It’s not arbitrary-it’s a compromise built on decades of pharmacokinetic data and real-world outcomes.
Think of it like a tolerance zone. If you’re manufacturing a gear that needs to fit perfectly, you don’t demand 100% precision-you allow a tiny margin of error. The 80-125% rule is that margin for drug absorption.
What Happens When a Drug Doesn’t Meet the Rule?
If a generic drug’s 90% confidence interval falls even slightly outside 80-125%, the FDA won’t approve it. That’s happened. Some generics have been rejected because their Cmax or AUC ratios were too far off-sometimes due to differences in formulation, fillers, or how the drug dissolves in the stomach.
But here’s the catch: even if a drug meets the 80-125% rule, it doesn’t mean it’s identical in every way. It just means the overall absorption profile is close enough that no clinically significant difference is expected. For most drugs, that’s fine. But for drugs with a narrow therapeutic index-like warfarin, levothyroxine, or certain anti-seizure medications-the stakes are higher.
In those cases, regulators sometimes tighten the range. The FDA now recommends a 90-111% interval for certain narrow therapeutic index drugs. That’s because even a 10% difference in blood levels could lead to serious side effects or loss of effectiveness. A patient on warfarin might bleed if the generic absorbs too quickly, or develop a clot if it absorbs too slowly. That’s why these drugs get extra scrutiny.
High-Variability Drugs and the Scaled Approach
Some drugs are naturally unpredictable in how they’re absorbed. These are called highly variable drugs. If a drug’s within-subject coefficient of variation is over 30%, the standard 80-125% rule becomes too strict. Why? Because even the brand-name drug varies a lot from one person to the next. If you compare two versions of a highly variable drug, their ratios might naturally fall outside 80-125%-not because one is worse, but because the drug itself is inconsistent.
That’s where scaled average bioequivalence (SABE) comes in. Instead of a fixed range, regulators adjust the acceptance limits based on how variable the reference drug is. For example, if a drug has very high variability, the acceptable range might stretch to 69.84-143.19% for Cmax. This approach is used by the EMA and WHO, and the FDA is moving toward adopting it more broadly.
It’s a smarter, more flexible system. It doesn’t force a one-size-fits-all rule on drugs that need individualized standards.
Common Misconceptions and Patient Fears
Despite decades of use, the 80-125% rule is still misunderstood. A 2022 survey found that 63% of community pharmacists believed generics could contain only 80% to 125% of the active ingredient. That’s wrong. In reality, both brand and generic pills must contain 95-105% of the labeled amount. The rule applies to how the body absorbs the drug-not how much is in the pill.
Patients hear “80%” and worry their medicine is weaker. Online forums are full of stories from people who switched to generics and felt different-sometimes due to changes in fillers, coatings, or how the pill breaks down. But those issues aren’t about bioequivalence. They’re about formulation differences that fall outside the scope of the 80-125% rule.
Post-market data tells a different story. Between 2003 and 2016, the FDA approved over 2,000 generic drugs. Only 0.34% needed label changes due to bioequivalence concerns after they hit the market. That’s a 99.66% success rate. For most people, switching to a generic is safe and effective.
Why This Rule Matters for Global Health
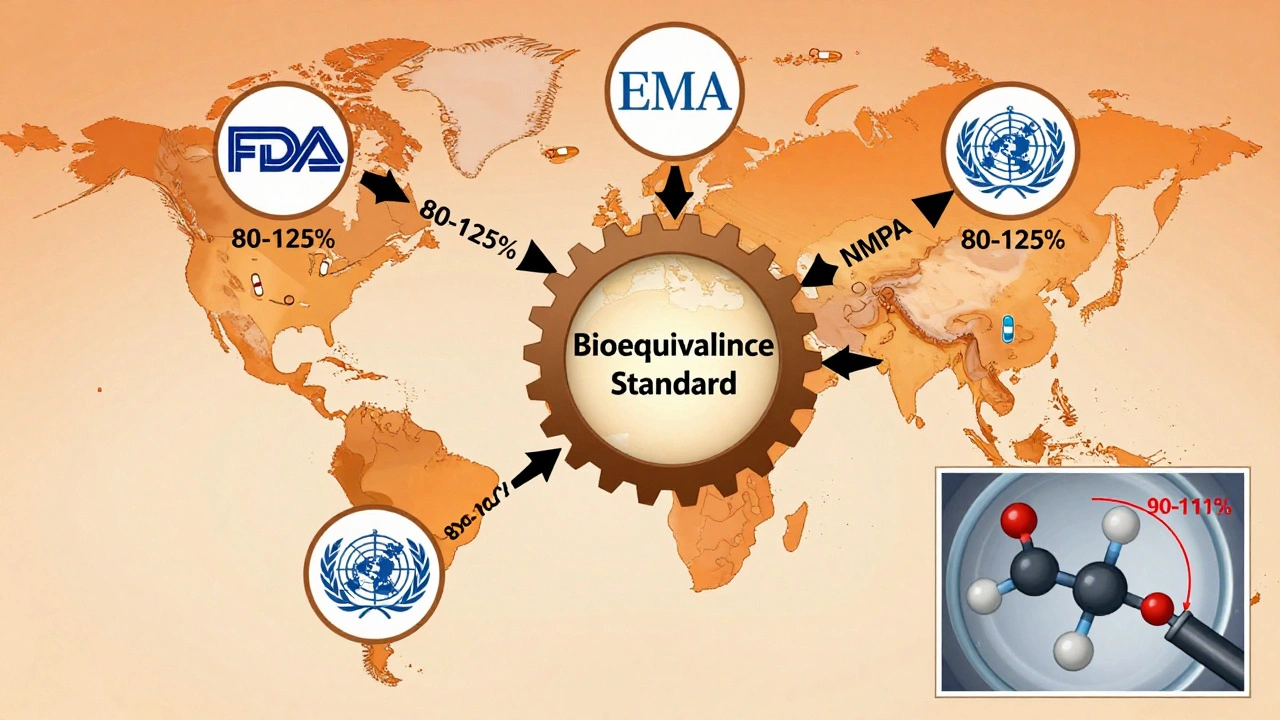
The 80-125% rule isn’t just an American standard. It’s used by the FDA, EMA, WHO, Health Canada, and China’s NMPA. That global alignment is why generic drugs are so affordable and widely available. Without this common standard, every country would have its own rules. Companies would need separate studies for each market. Development costs would skyrocket. And patients in low-income countries might never get access to life-saving generics.
Since the Hatch-Waxman Act of 1984, the U.S. has approved over 14,000 generic drugs. Today, 90% of prescriptions in the U.S. are filled with generics-but they make up only 23% of total drug spending. That’s billions saved every year.
That savings comes from the 80-125% rule. It lets companies skip expensive clinical trials. Instead, they run smaller, faster bioequivalence studies with healthy volunteers. Those studies cost $2-5 million and take 18-24 months-still a lot, but a fraction of the $1 billion+ it takes to develop a new brand drug.
What’s Next for Bioequivalence?
The rule isn’t static. The FDA is investing $15 million over the next few years to explore model-informed bioequivalence-using computer simulations to predict how a drug behaves in the body, instead of relying solely on human studies. That could reduce the need for clinical trials altogether for some drugs.
Researchers are also looking at pharmacogenomics. What if your genes affect how you absorb a drug? Future bioequivalence standards might need to account for genetic differences. A drug that works fine for most people might need a different standard for those with slow-metabolizing variants of CYP3A4 or CYP2D6 enzymes.
For complex products-like inhalers, topical creams, or extended-release pills-the 80-125% rule doesn’t always fit. That’s why the FDA launched its Complex Generics Initiative in 2018. These products don’t dissolve the same way in the body, so traditional PK measurements can’t capture their behavior. New methods, like in vitro-in vivo correlation (IVIVC), are being tested to replace or supplement the current standard.
But for now, the 80-125% rule remains the backbone of generic drug approval. It’s not perfect. It’s not always precise. But it’s proven. Over 40 years of data show that when a generic passes this test, it works just like the brand.
Bottom Line: Trust the Process
If your doctor prescribes a generic, you can be confident it’s been tested rigorously. The 80-125% rule isn’t a loophole-it’s a carefully designed safety net. It doesn’t guarantee identical pills. But it does guarantee that your body will get the same amount of medicine, at the same rate, as the brand-name version.
For the vast majority of drugs, that’s all you need.
Gwyneth Agnes
December 6, 2025 AT 17:0980-125% is just a statistical loophole dressed up as science.
Priya Ranjan
December 7, 2025 AT 10:05You think that's bad? In India, we've seen generics made in back-alley labs that barely dissolve. The 80-125% rule is a joke when you're dealing with suppliers who don't even know what AUC stands for. Regulators are asleep at the wheel, and patients are paying the price with their health. This isn't science-it's corporate convenience wrapped in math.
Ashish Vazirani
December 9, 2025 AT 09:01Let me tell you something-this whole system was designed by American pharma giants to crush competition! The 80-125% rule? A Trojan horse! They let in cheap generics from India and China, then act shocked when someone has a bad reaction. Meanwhile, our own drugmakers are forced to spend millions just to prove they’re 'close enough'-while foreign companies cut corners with fillers that haven’t been tested since the 90s! This isn’t regulation-it’s economic colonialism disguised as bioequivalence!
pallavi khushwani
December 10, 2025 AT 06:38It’s funny how we treat medicine like a math problem when it’s really about human bodies. The 80-125% rule works for most people, sure-but what about the ones who feel different after switching? Their experience isn’t invalid just because the numbers say they shouldn’t. Maybe the real question isn’t whether the drug is bioequivalent, but whether we’re okay with treating patients as statistical noise. I’ve seen people cry because their seizure control slipped after a generic switch-and the system just shrugs and says, 'It’s within range.' That’s not science. That’s surrender.
Katie O'Connell
December 11, 2025 AT 13:59While the statistical methodology underlying the 80-125% bioequivalence criterion is indeed grounded in robust pharmacokinetic principles, one cannot overlook the epistemological limitations inherent in reducing complex physiological responses to geometric mean ratios. The reliance upon healthy volunteers, who exhibit markedly different metabolic profiles from the target patient populations-particularly the elderly, renally impaired, or polypharmaceutical individuals-introduces a significant translational gap. Moreover, the implicit assumption of linear pharmacokinetics across all therapeutic classes is demonstrably untenable in the context of highly variable drugs or complex delivery systems. Consequently, while the framework has proven pragmatically useful, its foundational premises warrant rigorous re-evaluation in light of emerging pharmacogenomic and systems pharmacology paradigms.
Jackie Petersen
December 12, 2025 AT 05:52They say it's safe... but what if the real reason they use 90% CI instead of 95% is so they can approve more generics and hide the fact that Big Pharma owns the FDA? I read a guy on Twitter who said his blood thinners started making him bleed after switching-and they told him it was 'within range.' What range? The range where they don't get sued? The 80-125% rule is a cover-up for corporate greed. And don't even get me started on how the FDA lets Chinese factories make our meds without real inspections.
brenda olvera
December 12, 2025 AT 15:25I love how this rule lets so many people get the medicine they need without breaking the bank. I’ve seen friends in rural towns who couldn’t afford brand-name drugs-and now they’re alive because of generics. It’s not perfect, but it’s one of the few things in healthcare that actually works for regular people. The fact that over 99% of these drugs are fine? That’s a win. Let’s not fix what isn’t broken. Keep the science, keep the savings, keep the hope.
Ibrahim Yakubu
December 13, 2025 AT 00:10Let me tell you about my cousin in Lagos-he took a generic antiretroviral that was 'bioequivalent'... and his viral load spiked. The lab report said it met the 80-125% rule. But the pill had a different coating. It didn’t dissolve right in his stomach. He nearly died. This isn’t just math-it’s life or death. And when regulators in the West say 'it’s fine,' they forget that not everyone has access to labs that can check blood levels. The rule works in theory. In practice? It’s a lottery. And too many people are losing.
Brooke Evers
December 14, 2025 AT 23:33I just want to say how deeply important this is-not just for science, but for real people. I’ve worked with patients who’ve been terrified to switch from brand to generic because they’ve heard horror stories. And honestly? I get it. But what I’ve learned over the years is that the 80-125% rule is one of the most carefully thought-out protections we have. It’s not about perfection-it’s about safety with practicality. The fact that we’ve approved over 14,000 generics and only had 0.34% issues? That’s not luck. That’s decades of research, debate, and refinement. When someone tells you generics are dangerous, I invite them to look at the data-not the anecdotes. Because behind every one of those 14,000 approvals is someone who got to live a little longer, a little cheaper, a little more peacefully. That’s worth protecting.