Stability and Shelf Life: How Generic Products Degrade and Why Safety Depends on Testing
 Jan, 18 2026
Jan, 18 2026
When you pick up a bottle of generic medication, you assume it works just like the brand-name version. But here’s the truth: generic drugs aren’t always the same when it comes to how long they stay safe and effective. The difference isn’t in the active ingredient-it’s in what’s around it. Fillers, coatings, moisture barriers, and even how it’s packaged can change how fast the drug breaks down. And when it degrades, it’s not just less effective-it can become unsafe.
What Does "Stability" Really Mean?
Stability isn’t just about whether a pill still looks like a pill. It’s about whether every chemical inside it is still doing what it’s supposed to do. The U.S. Food and Drug Administration (FDA) defines stability as the ability of a product to maintain its chemical, physical, and microbiological properties over time. That means:- The active ingredient hasn’t broken down into harmful substances
- The pill still dissolves properly in your body
- No mold or bacteria has grown inside the container
- The dose you get is still accurate-no more, no less
How Do Drugs Actually Break Down?
Drugs don’t just expire because they’re old. They degrade because of chemistry. Heat, light, moisture, and oxygen all attack the molecules in a medication. Here’s how it happens:- Chemical degradation: Active ingredients break apart into impurities. For example, some antibiotics turn into toxic byproducts when exposed to moisture. The FDA requires that unknown impurities stay below 0.1%-any higher, and the product is considered unsafe.
- Physical degradation: Tablets crack, capsules soften, suspensions clump. In nanoparticle drugs, like those used for cystic fibrosis, particles must stay under 200 nanometers. If they clump up past that size, they can’t reach the lungs properly. One study showed a 40% drop in effectiveness when particles grew beyond this threshold.
- Microbiological degradation: Even non-sterile products can grow mold or bacteria if preservatives fail. Water activity (aw) and pH levels control this. A 2022 survey found that 41.3% of drug recalls linked to stability issues were caused by preservative systems breaking down due to moisture shifts.
- Functional degradation: Inhalers, patches, and injectables must deliver the exact dose every time. If the metering system clogs or the adhesive weakens, the patient gets too little-or too much.
Why Accelerated Testing Can Lie to You
To save time, manufacturers test drugs under extreme conditions-like 40°C (104°F) and 75% humidity-for just six months. They then use math to predict what will happen over two or three years. Sounds smart, right? It’s not always. In 2023, a quality assurance professional on a pharmaceutical forum shared a nightmare: their team ran accelerated tests on a new generic product. Nothing changed. They approved it. Then, after 24 months in real-world storage, the tablets started crystallizing. The drug lost potency. The batch was recalled. Cost? $250,000 and 18 months of lost time. Why? Because the degradation pathway at 40°C was different from what happened at room temperature. High heat can trigger one kind of breakdown, while slow moisture exposure triggers another. The same product might survive a hot lab test but fail in a humid bathroom cabinet. The FDA’s own guidelines say you can’t just extrapolate from accelerated data unless you’ve proven the degradation mechanism is the same. But many companies skip that step. And regulators are catching on. In 2022, 80% of FDA Form 483 observations related to stability testing were about poor documentation-like writing “room temperature” instead of recording actual storage data.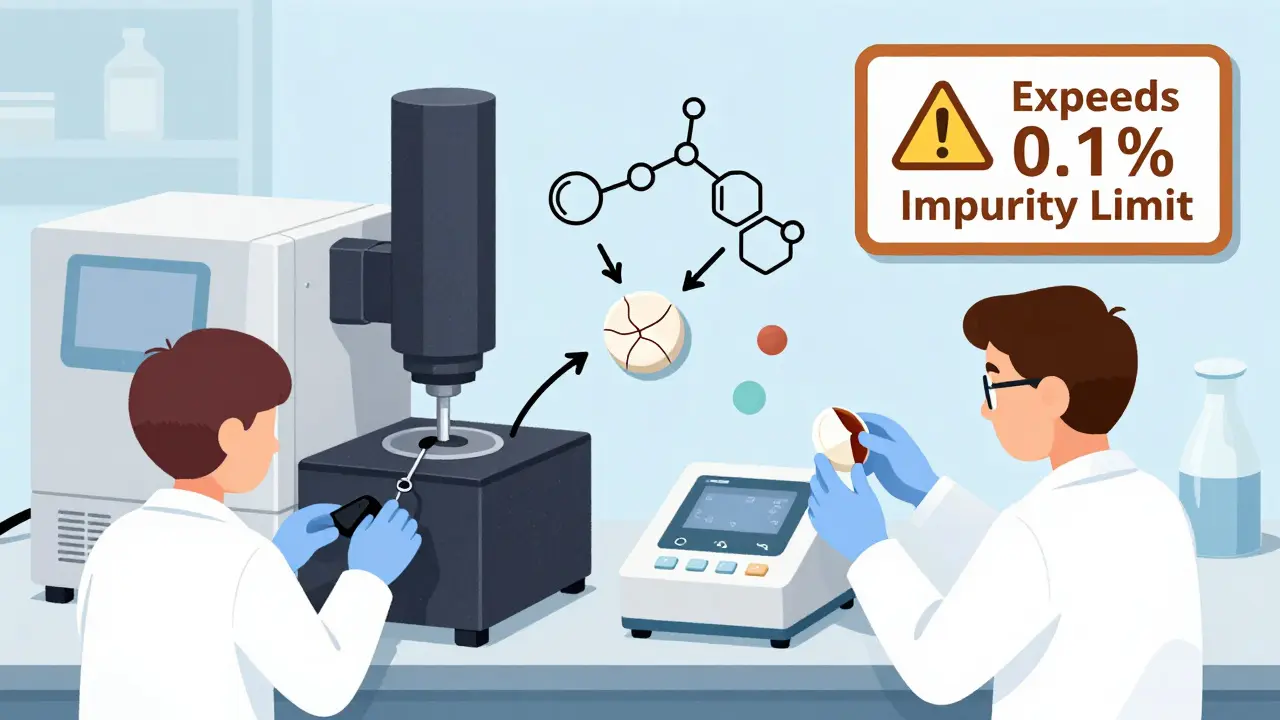
Storage Conditions Matter More Than You Think
“Room temperature” isn’t a number. It’s a range. The U.S. Pharmacopeia (USP) says 15-30°C (59-86°F). But research shows the ideal reference point for stability studies is 24-25°C. That’s not a coincidence-it’s the average temperature of a typical home or pharmacy shelf. But here’s the problem: most people don’t store medicine there. They keep it in the bathroom, on a windowsill, or in a hot car. A 2022 MIT study projected that by 2050, rising global temperatures will cause warehouses and homes to exceed 30°C for more than 87 days a year in major distribution hubs. That’s enough to shorten the shelf life of many drugs by nearly half a year. And it’s worse for generics. Brand-name companies often invest in climate-controlled packaging. Generics? They cut costs. A 2021 WHO report found that 28.7% of medicines in low-income countries failed stability tests-not because they were fake, but because they were stored in uncontrolled conditions during transport and sale.Why Generic Drugs Are More Vulnerable
Generic drugs are required to be “bioequivalent”-meaning they deliver the same amount of active ingredient into the bloodstream. But they’re not required to match the brand’s excipients (inactive ingredients) or manufacturing process. That’s a problem because excipients control stability. A coating that keeps moisture out in the brand-name version might be replaced with a cheaper alternative in the generic. A preservative system that lasts three years might be swapped for one that lasts 18 months. Texas A&M researchers found that even small changes in filler composition altered how quickly a drug degraded. One generic version of a common antihypertensive drug degraded 30% faster than its brand counterpart-despite passing bioequivalence tests. And because generics are often made by multiple manufacturers, you might get a different version each time you refill your prescription. One batch might be stable. The next might not be.
What You Can Do to Stay Safe
You can’t test your medicine in a lab. But you can take steps to protect yourself:- Check the expiration date. Don’t use medicine past that date-even if it looks fine.
- Store it right. Keep pills in a cool, dry place. Not the bathroom. Not the dashboard. A bedroom drawer is better.
- Watch for changes. If a tablet changes color, smells odd, or crumbles, don’t take it. If a liquid looks cloudy or has particles, throw it out.
- Ask your pharmacist. If you switch to a new generic and notice side effects or reduced effectiveness, ask if it’s the same formulation as before.
The Future: Faster, Smarter Testing
The industry is changing. The FDA’s new Continuous Manufacturing Stability Testing (CMST) pilot showed shelf life can be determined 40% faster for drugs made in continuous lines-not batch by batch. The ICH’s Q12 guideline, effective in late 2023, lets companies make changes to stability protocols after approval without reapplying-giving more flexibility. Companies like Amgen and Merck are using Risk-Based Predictive Stability (RBPS) tools that combine real-time sensor data with AI to predict degradation. In trials, these tools cut testing time by 30%. But regulators still don’t fully accept them. Why? Because they’re complex. And medicine safety isn’t a place for guesswork. Still, the trend is clear: the future of stability testing won’t be about waiting three years. It’ll be about understanding the science behind the breakdown-and stopping it before it starts.Bottom Line: Don’t Assume Generic Means Identical
Generic drugs save billions of dollars. That’s good. But assuming they’re identical in every way-especially in how long they last-is dangerous. Stability isn’t just a regulatory checkbox. It’s the difference between healing and harm. If your medication doesn’t work like it used to, or you notice strange side effects after switching generics, it might not be your body changing. It might be the pill. Trust your instincts. Check the date. Store it right. Ask questions. Your health depends on more than just the active ingredient. It depends on whether the whole product stayed intact.Can expired generic medications become dangerous?
Yes. While many expired medications simply lose potency, some can break down into toxic compounds. For example, tetracycline antibiotics can degrade into substances that damage the kidneys. Even non-antibiotic drugs like insulin or nitroglycerin can become ineffective or unstable after expiration, leading to treatment failure or adverse reactions.
Why do some generic drugs expire faster than brand-name ones?
Generics use different inactive ingredients (excipients) and packaging to cut costs. These differences affect how well the product resists moisture, heat, and light. A brand-name drug might use a specialized moisture barrier; a generic might use a thinner film. Even small changes can lead to faster degradation, as seen in the 2020 FDA study on levothyroxine generics.
Is it safe to take a generic drug that’s one month past its expiration date?
For most stable drugs like acetaminophen or ibuprofen, taking it one month past expiration is unlikely to cause harm-but it may be less effective. For critical medications like heart pills, insulin, or epinephrine, don’t take it. The risk of failure isn’t worth it. Always consult your pharmacist before using expired medicine.
How do I know if my medication has degraded?
Look for visible signs: tablets that crumble, change color, or develop spots; capsules that stick together or leak; liquids that cloud or separate. Smell matters too-medications that smell rancid or chemical aren’t safe. If you’re unsure, don’t guess. Return it to your pharmacy for disposal.
Does refrigeration always extend shelf life?
Not always. Some medications, like certain antibiotics or tablets, can degrade faster when refrigerated due to moisture condensation. Always follow the storage instructions on the label. If it says “store at room temperature,” don’t refrigerate unless your pharmacist advises otherwise.
Are there any regulations that ensure generic drugs have the same shelf life as brand-name drugs?
No. The FDA requires generics to be bioequivalent, meaning they deliver the same amount of active ingredient. But they don’t require matching excipients, packaging, or stability profiles. Manufacturers must prove their generic is stable for its labeled shelf life, but that period can-and often does-differ from the brand-name product.
Jacob Hill
January 19, 2026 AT 19:16Wow, this is eye-opening. I never thought about how the filler in generics could make or break a pill’s stability. I’ve been storing my blood pressure med in the bathroom-ugh. I’m moving it to a drawer tonight. And honestly? I’m gonna start asking my pharmacist which manufacturer made each batch. If one version makes me feel weird and another doesn’t-it’s not me. It’s the pill.
Valerie DeLoach
January 20, 2026 AT 21:20Thank you for writing this with such clarity. It’s easy to assume that ‘bioequivalent’ means ‘identical,’ but chemistry doesn’t care about labels-it cares about molecular behavior. The fact that moisture barriers, not active ingredients, are the silent killers here is terrifying. And yet, we’re expected to trust that a $5 pill is just as safe as a $50 one. We need transparency-not just in labeling, but in packaging specs. This isn’t about cost-cutting; it’s about patient safety.
Christi Steinbeck
January 22, 2026 AT 03:48STOP. RIGHT. NOW. If you’re keeping your meds in the bathroom, you’re basically playing Russian roulette with your health. I’ve seen patients show up with crumbly pills that smell like wet socks-yes, that’s a thing-and then wonder why their thyroid meds ‘stopped working.’ It’s not your body. It’s the humidity. Don’t be that person. Buy a little plastic container. Put it in your bedroom. Live longer.
Lewis Yeaple
January 23, 2026 AT 00:21While the article presents a compelling case regarding the variability in generic drug stability, it is imperative to note that the U.S. Food and Drug Administration mandates that all approved generics meet stringent bioequivalence standards. Although excipient variation exists, regulatory oversight ensures that degradation products remain within acceptable limits. To suggest that generics are inherently less safe is misleading without acknowledging the rigorous post-market surveillance systems in place.
Jackson Doughart
January 24, 2026 AT 13:33There’s a quiet tragedy here: people assume medicine is like bread-just don’t eat it past the date. But pills aren’t bread. They’re complex chemical systems. And when the coating fails, or the preservative evaporates, the degradation isn’t linear-it’s exponential. I’ve worked in pharmacy for twenty years. I’ve seen people collapse because their insulin degraded in a hot car. It’s not fearmongering. It’s physics. And we’re not educating patients enough.
Malikah Rajap
January 26, 2026 AT 10:58Okay, but like… why do we even have generics?? Like, if they’re gonna be unstable and weird and sometimes make you feel like a zombie, why not just pay for the brand? I mean, I get it, money, but my anxiety meds went from ‘calm me down’ to ‘why am I crying in the shower’ after a refill… and it was a different generic. So now I just… pay more. My mental health is worth $15 more a month. Not sorry.
sujit paul
January 27, 2026 AT 07:33Mark my words: this is all part of the globalist pharmaceutical cartel's plan. The FDA, WHO, and Big Pharma collude to push cheap generics so that the masses become dependent on unstable drugs-then when they get sick, they need more pills, more doctors, more control. The real reason generics degrade faster? Because they're designed to. The moisture? The packaging? All intentional. They want you sick. And they want you hooked. Check the batch numbers. They all start with the same code. Coincidence? I think not.
Tracy Howard
January 27, 2026 AT 13:33Can we just admit that American healthcare is a joke? We let Indian and Chinese factories make our life-saving drugs with packaging that can’t even handle a humid day. Meanwhile, Canada and the EU have stricter stability standards. Why do we accept this? Because we’re too lazy to demand better. We’d rather save $10 and risk our lives. Shameful. If you’re using a generic, you’re basically gambling with your organs.
Aman Kumar
January 28, 2026 AT 12:50Let’s be brutally honest: the entire generic drug ecosystem is a regulatory failure disguised as cost-efficiency. Bioequivalence is a proxy metric-a statistical mirage. Stability is not measured at the population level; it’s measured at the molecular level, and manufacturers exploit loopholes in excipient substitution protocols. The 2020 levothyroxine case? That’s the tip of the iceberg. We need mandatory full-spectrum stability disclosure-batch-by-batch, excipient-by-excipient. Until then, we’re not patients. We’re test subjects.