Patent Term Restoration (PTE): How It Extends Drug Patent Life
 Feb, 11 2026
Feb, 11 2026
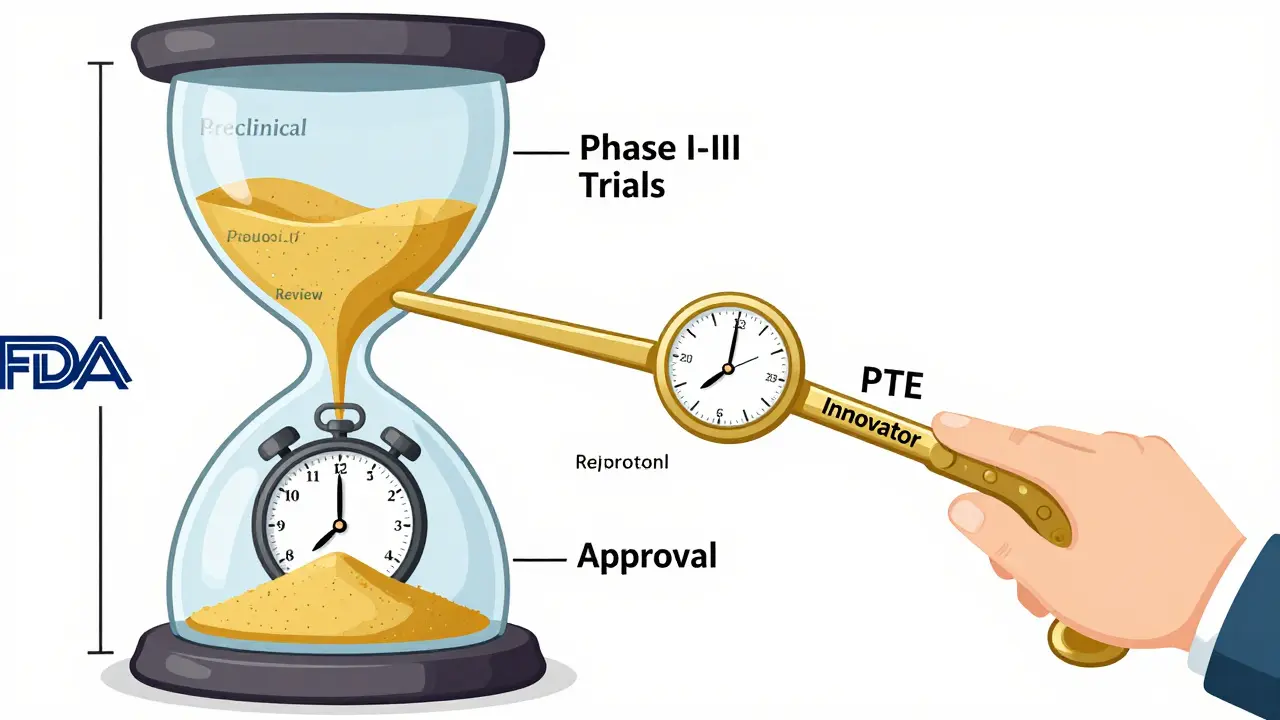
When a pharmaceutical company spends over a decade and $2.6 billion developing a new drug, it doesn’t just need a patent - it needs patent term restoration to survive. Without it, most life-saving medicines would lose protection before they even hit the market. That’s because the clock on a patent starts ticking the day the application is filed, not when the FDA gives final approval. And that approval process? It can take 10 to 15 years. By the time a drug is cleared for sale, half the patent life is already gone. Patent Term Restoration (PTE) fixes that. It’s not a loophole. It’s a legal reset button built into U.S. law to make sure innovators aren’t punished for regulatory delays.
How PTE Works: The Hatch-Waxman Act and the Real Math
The system started in 1984 with the Hatch-Waxman Act, named after Senator Orrin Hatch and Representative Henry Waxman. It wasn’t meant to give drug companies forever exclusivity. It was meant to balance two goals: let generics enter the market faster, and make sure innovators get a fair shot at recouping their investment. The math behind PTE isn’t simple, but here’s the core idea:
- Your patent expires 20 years from the filing date - standard rule.
- Time spent waiting for FDA approval counts against that 20 years.
- PTE adds back some of that lost time - up to five years max.
- But the total protected time after approval can’t go beyond 14 years.
For example: If a drug was filed in 2010, approved in 2020, and spent 8 years in FDA review, PTE could add up to 5 years of protection - meaning the patent would now expire in 2025 instead of 2030. But if the original patent was filed in 2015 and approved in 2025, the extension would be smaller because the 14-year cap kicks in. The system is designed to reward delay, not inflate profits.
Who Gets PTE? Not Everything Qualifies
PTE doesn’t apply to every patent. It’s limited to products that go through FDA review before being sold. That means:
- Human drugs - yes
- Animal drugs - yes (since 1988)
- Medical devices - yes
- Food additives - yes
- Color additives - yes
- Software, apps, or medical diagnostics without FDA review - no
Even within those categories, only one patent per product can get extended. And it has to be the patent that covers the product as approved - not a secondary patent on a new dosage form or method of use. The FDA and USPTO cross-check this carefully. In 2023, 312 PTE applications were filed. Only about 87% were approved. The rest? Mostly denied because they tried to extend the wrong patent.
The Application Process: 60 Days to File - No Extensions
Timing is everything. You have exactly 60 days from the date your product gets FDA approval to file for PTE. Miss that window? You lose it. Forever. No grace period. No exceptions. That’s why pharmaceutical companies run internal countdowns like a rocket launch. The paperwork is brutal. You need:
- Full FDA regulatory review records
- Every communication with the FDA - emails, letters, meeting notes
- Proof you didn’t delay the process - called “due diligence”
- Exact dates for every submission, response, and revision
One major company lost a $200 million extension in 2022 because their internal team didn’t log a single email exchange during a 45-day FDA request for additional data. The USPTO said: “No continuous progress. No extension.” The company had to drop the patent early. That’s not rare. About 12.7% of applications get denied - almost all for poor documentation.

Interim Extensions: A Lifeline for Late-Stage Drugs
What if your patent expires in six months, but the FDA hasn’t approved your drug yet? You’re out of luck - unless you ask for an interim extension. This is a temporary fix that lets you keep patent rights while waiting for final approval. You can apply anytime between six months and 15 days before your patent expires. The FDA reviews it fast - usually within 30 days. If granted, you get protection until the final decision. It’s not automatic. But for companies with drugs in Phase III trials, it’s often the only thing standing between a breakthrough and a financial collapse.
Why PTE Is Controversial - And How It’s Being Used
Here’s the uncomfortable truth: PTE was meant to protect original compound patents. But today, 78% of PTE applications involve secondary patents - things like new formulations, delivery methods, or dosing schedules. A 2022 study in the Journal of the American Medical Association found that after a drug gets its PTE, companies often file 3-5 more patents on minor changes. This creates what critics call “patent thickets” - a maze of overlapping protections that block generics even after the original patent should have expired.
Take Eli Lilly’s diabetes drug, Humalog. The original patent expired in 2015. But through three PTEs on reformulated versions, the company kept market exclusivity until 2024. That’s nine extra years of monopoly pricing. The Congressional Budget Office estimates PTE adds $4.2 billion a year to U.S. drug spending. The FTC says drugs with PTE hold 92% of the market during the extension - compared to 37% after generics arrive.
But here’s the flip side: Without PTE, many drugs would never get developed. Biotech startups don’t have deep pockets. If they can’t get even five extra years of protection, investors walk away. In 2023, 34% of PTE applications were for biologics - complex drugs made from living cells. These take even longer to approve. PTE is often the only reason these drugs exist at all.
What’s Changing in 2025-2026?
The system is under pressure. In January 2024, the FDA issued new guidance on “due diligence,” requiring companies to show day-by-day progress - not just milestone dates. The USPTO’s denial rate is climbing. A 2024 Federal Circuit ruling made it harder to prove you didn’t cause delays. And the FDA plans to launch a digital submission portal in Q2 2026. That will cut processing time from 217 days to under 90.
Meanwhile, Congress is debating the Preserve Access to Affordable Generics and Biosimilars Act. If passed, it would ban PTE for secondary patents that don’t involve the active ingredient. That could slash future extensions by 60%. But for now, PTE remains a powerful tool - one that’s being used more than ever. In 2023, 312 applications were filed - up 7.3% from 2022. Biologics, gene therapies, and regenerative medicine are driving the growth.
What You Need to Know If You’re in Pharma
If you’re a patent attorney, a regulatory affairs manager, or a startup founder:
- Start tracking FDA timeline the day you file your patent.
- Assign one person to log every FDA interaction - no exceptions.
- Don’t wait until approval to start your PTE application. Begin drafting 12 months ahead.
- Know which patent you’re extending. Only one per product. Choose wisely.
- Use the FDA’s free guidance portal ([email protected]). They answered 1,842 questions in 2023 - average response time: 72 hours.
PTE isn’t magic. It’s paperwork. It’s precision. It’s patience. And for the right product, it’s worth billions.
Can a patent be extended more than once for the same drug?
No. Only one patent per product can receive a patent term restoration. Even if a company has multiple patents covering different aspects of the same drug - like formulation, method of use, or delivery system - only one can be extended. The FDA and USPTO cross-check this using the Orange Book. Choosing the wrong patent to extend can cost a company millions in lost revenue.
What happens if I miss the 60-day deadline to apply for PTE?
You lose the right forever. There are no extensions, no grace periods, and no exceptions. The law is strict: 60 days from FDA approval date. Many companies lose PTE because their legal team doesn’t coordinate with regulatory affairs. If your drug is approved on March 1, your PTE application must be filed by April 30. Set calendar alerts. Assign backups. Treat this like a court deadline.
Does PTE apply to generic drugs?
No. PTE is only available to the original innovator who holds the patent and went through the full FDA approval process. Generics don’t get PTE - they rely on the expiration of the original patent. In fact, one of the goals of the Hatch-Waxman Act was to speed up generic entry by balancing patent extensions with faster approval for generics. PTE delays generics. It doesn’t help them.
How long does the PTE application process take?
On average, it takes 217 days from submission to decision, according to FDA’s 2023 report. But this can vary. If your application is complete and well-documented, it might be approved in 120 days. If the USPTO has questions or the FDA needs more data, it can stretch to over a year. The biggest delays come from incomplete due diligence records - missing emails, unlogged meetings, or vague timelines.
Can PTE be used for medical devices or diagnostics?
Yes - but only if the device requires FDA premarket approval (PMA) or a 510(k) clearance. Simple diagnostic tests that don’t need FDA review don’t qualify. For example, a new implantable heart monitor that went through a full PMA process can get PTE. A blood test kit that only needed a 510(k) clearance can also qualify. But a smartphone app that analyzes heart rate data without FDA clearance? No. The key is whether the product had to be reviewed by the FDA before being sold.