Generic Drugs: What They Are and How They Work
 Jan, 27 2026
Jan, 27 2026
When you pick up a prescription, you might see two names on the label: one you recognize, like Lipitor, and another that looks unfamiliar, like atorvastatin. The second one is a generic drug. It’s not a cheaper version or a knockoff-it’s the exact same medicine, just without the brand name. And for most people, it works just as well, for a fraction of the cost.
What Exactly Is a Generic Drug?
A generic drug contains the same active ingredient as the brand-name version. That means if your doctor prescribes a brand-name drug like Prozac (fluoxetine), the generic version is just fluoxetine. The chemical structure, how it works in your body, and the dose are identical. The U.S. Food and Drug Administration (FDA) requires that generics meet the same strict standards for safety, strength, purity, and quality as the original brand.
Generics become available only after the brand-name drug’s patent expires. Patents typically last 20 years from the date they’re filed, but because clinical testing takes years before approval, the actual market exclusivity is often closer to 10-12 years. Once that window closes, other companies can apply to make the same drug. This process is called the Abbreviated New Drug Application (ANDA), created by the Hatch-Waxman Act of 1984. It lets generic manufacturers skip expensive animal and human trials because they only need to prove their version works the same way as the original.
How Do We Know Generics Work the Same Way?
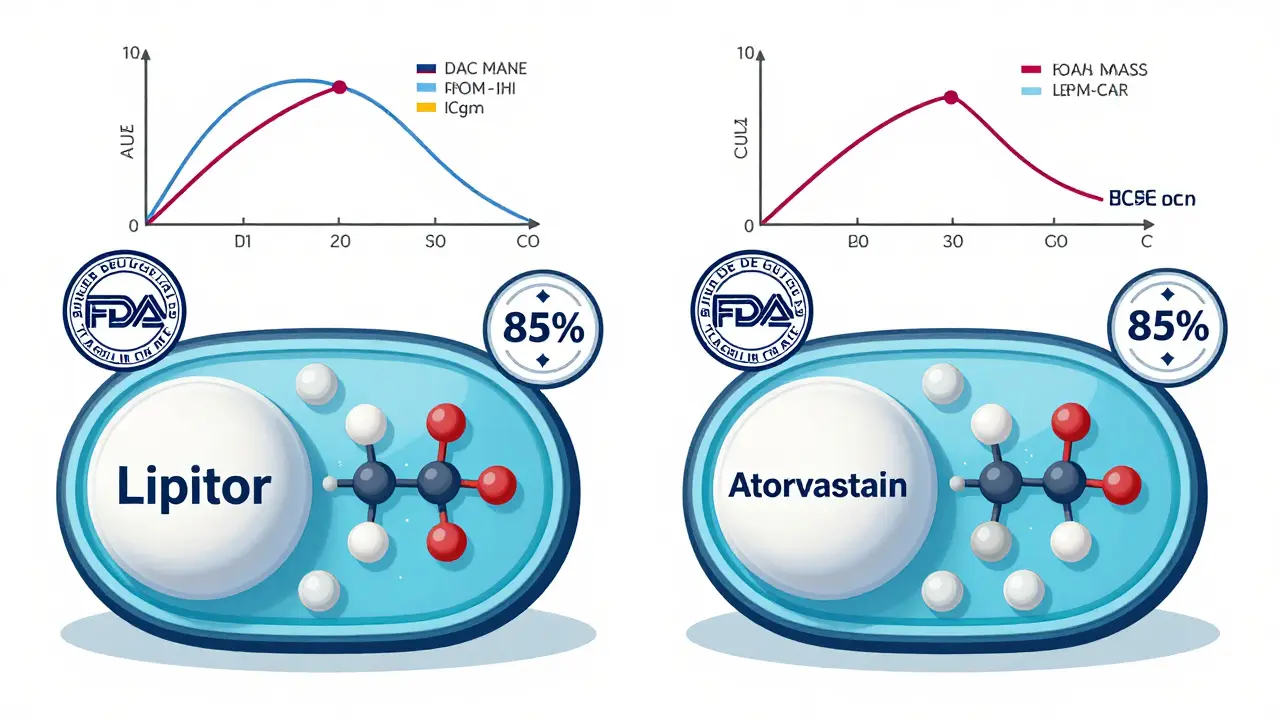
The biggest concern people have is whether generics are truly equivalent. The answer lies in bioequivalence. This isn’t just a buzzword-it’s a scientific requirement. To get FDA approval, a generic must deliver the same amount of active ingredient into your bloodstream at the same rate as the brand-name drug. The FDA uses precise measurements called AUC (area under the curve) and Cmax (peak concentration) to compare how the body absorbs the drug.
The standard? The generic’s absorption must fall within 80% to 125% of the brand’s. That’s a tight range. For example, if the brand delivers 100 units of the drug into your blood over time, the generic must deliver between 80 and 125 units. Any more variation than that, and it wouldn’t be approved. This isn’t theoretical-it’s tested in real people, usually 24 to 36 healthy volunteers, under controlled conditions.
Studies back this up. The Institute of Medicine reviewed 38 clinical trials on generic cardiovascular drugs and found no meaningful difference in effectiveness compared to brand names. The American College of Physicians, the American Medical Association, and the FDA all agree: generics are therapeutically equivalent.
What’s Different About Generics?
If the active ingredient is the same, why do generics look different? That’s because of inactive ingredients-things like dyes, fillers, and coatings. These don’t affect how the drug works, but they’re changed to avoid trademark infringement. So your generic version of Viagra might be a blue oval instead of a diamond-shaped pill, and it might taste slightly different. But the medicine inside? Identical.
Even the manufacturing process is held to the same standard. The FDA inspects generic drug factories just like brand-name ones-about 3,500 inspections a year worldwide. These aren’t scheduled visits; they’re unannounced. Inspectors check everything: how ingredients are mixed, how pills are compressed, how quality is tested. The rules for cleanliness, documentation, and testing are exactly the same.
There’s one small exception: some drugs have a narrow therapeutic index. That means even tiny changes in blood levels can cause problems. Examples include warfarin (a blood thinner), levothyroxine (for thyroid issues), and some epilepsy drugs. For these, doctors may prefer to stick with one version-brand or generic-because switching could require more frequent monitoring. But even here, the FDA approves generics only if they meet bioequivalence standards. The risk isn’t from the drug itself-it’s from human error or inconsistent switching.

Why Are Generics So Much Cheaper?
Brand-name drugs cost a lot because the company that invented them had to pay for research, clinical trials, marketing, and patent protection. The average cost to bring a new drug to market? Around $2.6 billion, according to research published in the Journal of Health Economics.
Generic manufacturers don’t have to repeat those steps. They don’t pay for the original research. They don’t run massive advertising campaigns. They don’t need to recoup billions in development costs. So they can sell the same medicine for 80% to 85% less. In some cases, when five or more generic makers enter the market, prices drop to just 9% of the original brand price.
The numbers speak for themselves. In 2022, 90.5% of all prescriptions filled in the U.S. were generics. Yet they made up only 13.1% of total drug spending. Over the past decade, generics saved the U.S. healthcare system an estimated $2.18 trillion.
What About Biosimilars? Are They the Same?
Not all drugs are simple chemicals. Some, like insulin or rheumatoid arthritis treatments, are made from living cells. These are called biologics. They’re too complex to copy exactly. So instead of generics, we have biosimilars.
Biosimilars are highly similar to the original biologic, but not identical. They require more testing and often cost less, but not as dramatically. While traditional generics save 80-85%, biosimilars typically save only 20-30%. That’s because producing them is still expensive and technically difficult. As of 2022, biosimilars had captured only about 31% of their potential market in approved uses. But that’s changing as more biologics lose patent protection.
How Are Generics Approved and Monitored?
The FDA’s Office of Generic Drugs reviews about 1,000 ANDAs each year. The average review time is 10 months. Once approved, the drug is added to the FDA’s Orange Book, which lists all approved generics and their brand-name equivalents. Pharmacists can legally substitute a generic unless the doctor writes “dispense as written.” That’s allowed in 49 states.
After approval, the FDA keeps watching. All drugs-brand and generic-are tracked for side effects through the MedWatch system. If a generic causes unexpected problems, the FDA can pull it. There’s no special treatment for generics; they’re held to the same post-market surveillance standards.
Still, challenges remain. In 2022, the FDA reported a 22% increase in drug shortages. Many of those were linked to manufacturing issues at generic drug plants, especially those overseas. About 80% of the active ingredients in U.S. generics come from India and China. That’s efficient-but it also makes the supply chain vulnerable. The White House has flagged this as a national security concern.
What’s Next for Generic Drugs?
More than 350 brand-name drugs with combined annual sales of $138 billion are set to lose patent protection between 2023 and 2027. That means a wave of new generics is coming. The FDA is preparing by speeding up reviews under its GDUFA III program and issuing more detailed guidance for complex drugs like inhalers, eye drops, and topical creams.
At the same time, some brand-name companies are launching their own generics-called authorized generics-to compete in the low-price market. These are made by the original manufacturer but sold under a different label. They’re often priced just slightly above the cheapest generic, but they give patients a familiar option.
What’s clear is this: generics aren’t a compromise. They’re a smart, safe, and proven way to get the medicine you need without paying a premium for a brand name.
Can I Trust My Generic Prescription?
Yes. If your doctor prescribes a generic, you can be confident it’s been tested, approved, and monitored just like the brand-name version. If you’re unsure, ask your pharmacist. They can tell you if your generic looks different from last time, and why. If you’ve had a bad experience switching-like feeling worse after changing pills-talk to your doctor. But don’t assume it’s the generic’s fault. Often, it’s something else: stress, diet, another medication, or even the placebo effect.
For most people, switching to a generic is one of the easiest ways to save money without sacrificing care. And with over 90% of prescriptions filled with generics in the U.S., you’re not alone. Millions of people rely on them every day. They’re not second-choice medicine. They’re the standard.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same safety and quality standards as brand-name drugs. They use the same active ingredients, are made in the same type of facilities, and are subject to the same inspections. The only differences are in inactive ingredients like color or shape, which don’t affect safety.
Why do generic drugs look different from brand-name drugs?
By law, generic drugs must look different from brand-name versions to avoid trademark infringement. That means the pill might be a different color, shape, or size. The active ingredient is the same, but the fillers, dyes, or coatings can vary. These changes don’t affect how the drug works.
Can I switch between brand-name and generic drugs safely?
For most drugs, yes. But for medications with a narrow therapeutic index-like warfarin, levothyroxine, or certain seizure drugs-small changes in blood levels can matter. Your doctor may recommend sticking with one version to avoid the need for frequent monitoring. Always talk to your doctor before switching.
Why are generic drugs so much cheaper?
Generic manufacturers don’t have to pay for the original research, clinical trials, or marketing that brand-name companies do. Once a patent expires, multiple companies can produce the same drug, driving prices down. On average, generics cost 80-85% less than brand-name versions.
Do generic drugs take longer to work?
No. The FDA requires generics to be bioequivalent, meaning they enter your bloodstream at the same rate and to the same extent as the brand-name drug. If the brand works in 30 minutes, so does the generic. Any delay you notice is likely due to other factors, like what you ate or how you took the pill.
Are all generic drugs made in the U.S.?
No. About 80% of the active ingredients in U.S. generic drugs come from facilities in India and China. The FDA inspects these facilities just like U.S.-based ones. The final product must meet the same standards regardless of where it’s made.
fiona vaz
January 28, 2026 AT 16:12I switched to generics years ago after my insurance stopped covering brand-name meds. My blood pressure hasn't budged, and I'm saving $40 a month. No side effects, no weird reactions-just the same results for less. If you're nervous, ask your pharmacist to compare the active ingredients. They're the same.